World's First 3D-Printed Titanium Cranial Implant Cleared By FDA
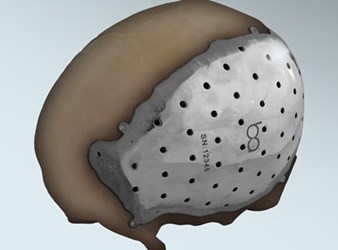
The U.S. Food and Drug Administration (FDA) has cleared the first 3D-printed cranial/craniofacial plate implant to be made from titanium. Brazil-based BioArchitects’ implant uses Arcam AB’s electron beam melting (EBM) technology to combine the precision and customization of 3D printing with the light weight and high tensile strength of titanium alloys.
When Oxford Performance Materials (OPM) announced the FDA clearance of its polymer-based craniofacial implant in 2014, CEO Scott DeFelice commented that there has been a “substantial unmet need” for personalized and economical solutions for facial reconstruction. By introducing an implant that is titanium-based, BioArchitects has made yet another contribution to that need.
“We are extremely proud to contribute to what we consider another major advance in the trend toward personalized medicine,” said Mark Ulrich, CEO of BioArchitects USA, in a press release. “We believe that this is yet another step toward what will ultimately become the new standard of care.”
The BioArchitects implant was designed to attach to existing bone with self-tapping titanium screws, replacing defective bone lost from congenital condition, disease, or trauma. Each implant is custom-made using MRI and CT scans from the individual patient, and 3D printed using Arcam’s EBM technology, which allows for extremely precise specifications by using electron beams to melt and shape powdered alloys.
According to Arcam, an increasingly competitive orthopedic market has pushed manufacturers to either innovate or reduce costs, but additive manufacturing allows for simultaneous product differentiation and cost reduction. Arcam CEO Magnus Rene said his company has been working with the orthopedic implant industry for over a decade to create new technologies.
“BioArchitects is a prime example of how innovative organizations are using EBM technology to advance biomedical surgeries that truly affect people’s lives,” said Rene in a press release.
Traditional titanium plates already on the market use separate attachments and metal brackets to hold the plate in place. By incorporating the attachment heads into the plate itself, surgeons and biomedical engineers can plan out their placement and ensure a better fit, said Ulrich in an interview with Design News. As part of BioArchitects’ FDA approval, they tested the self-tapping screws against existing titanium brackets and found them to be three to four times stronger.
Analysts from iData Research told MDO that the trauma device market will be worth $8 billion by 2020, and the increasing popularity of titanium will contribute to that growth. “The increased associated structural strength involved with using titanium materials has penetrated the majority of the trauma markets,” said analysts, citing titanium’s higher biocompatibility and light-weight flexibility as compared to stainless steel.
Last year, a team of German scientists implanted the world’s first 3D-printed titanium cervical implant, and an Australia-based Anatomics designed a titanium replacement sternum and rib cage with sockets to fit onto existing bone.
Recently, MedShape announced FDA clearance of its 3D-printed titanium bone tether plate, a solution to correct hallux valgus (bunion) deformities in the feet.
