Tumor-On-A-Chip Developed For Therapeutic Testing
By Chuck Seegert, Ph.D.
In some cases, nanoparticle therapeutics haven’t been deployed in the fight against cancer because their efficacy can’t reasonably be demonstrated. Now, however, a test system developed by Purdue University researchers could enable effectiveness testing for cancer-targeting nanoparticles and drugs.
Finding exploitable differences between healthy tissue and cancerous tumors is generally the goal of any therapeutic treatment option. For example, chemotherapy often targets rapidly dividing cells like those seen in tumors. Unfortunately, there are other healthy tissues in the body, like hair follicles and epithelial linings of the gastrointestinal tract, that are affected too. So, this exploitable difference isn’t completely isolated to the cancer cells.
Another potentially exploitable difference is tumor pore size, according to a recent press release from Purdue University. Often pores in tumors are poorly formed and thus larger, which may allow selective uptake of nanoparticles. Pores in normal tissue, being too small, would not absorb the drugs being delivered.
"It was thought that if nanoparticles were designed to be the right size they could selectively move toward only the tumor," said Bumsoo Han, a Purdue University associate professor of mechanical engineering, in the press release.
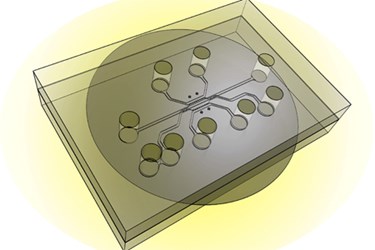
This still may not work, however, as interstitial pressure is often greater in tumors and, therefore, leads to an environment that pushes out drugs or imaging agents — resulting in minimal uptake. To better understand these complex scenarios, a team led by professor Han developed a tumor-microenvironment-on-chip (T-MOC) device.
Research suggesting the T-MOC device can replicate the complex conditions around a tumor is published in a study in the Journal of Controlled Release. The in vitro model is composed of a 3D structure of microfluidic channels that houses tumor and endothelial cells in culture. Perfusion of fluid allows a simulation and manipulation of artificial interstitial pressures. Pore sizes can also be tailored for certain experiments to give detailed information about how nanoparticles are transported in the device.
This new device may offer an alternative approach to testing new nanoparticle-based drug delivery methods. According to the press release, the team expects future work to center around personalizing treatment options by culturing patient-specific cells in the device and testing drug efficacy.
Using microfluidic devices for cancer research has also been useful for diagnostics. Recently in an article published on Med Device Online, a diagnostic apparatus was discussed that used a microfluidic structure to identify pancreatic cancer.
Image Credit: Purdue University photo/Altug Ozcelikkale, Bumsoo Han
