Philips Launches Wearable Diagnostic Device To Combat Children's Pneumonia
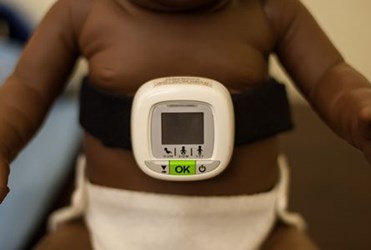
To honor World Pneumonia Day, Philips has announced the upcoming launch of a diagnostic device that can monitor and measure breath sounds of patients suspected to have pneumonia, and innovation that could have an impact on healthcare efforts in low-resource countries. The Children’s Automated Respiration Monitor (CARM) will join a global initiative to end preventable childhood deaths by 2025.
The World Health Organization (WHO) reported that pneumonia is responsible for an estimated 15 percent of deaths in children younger than 5 years, close to a million children in 2015 alone. Strains of pneumonia caused by bacteria — the most common among children — can be effectively treated with antibiotics, but only if the children are properly diagnosed and treated.
In 2005, Philips launched Philanthropy by Design, a program designed to direct resources and creative solutions toward some of the world’s biggest healthcare challenges, and sought advice from leading non-governmental organizations (NGOs) on where and how they might make the most impact.
A common symptom of childhood pneumonia is fast breathing, and healthcare workers in low-resource areas diagnose the disease by using an acute respiratory infection (ARI) timer and counting breaths—a method that is not always reliable. One of Philips’ philanthropic objectives has been to develop a device that could make diagnosing pneumonia less subjective.
Instead of listening for breaths, the CARM uses accelerators to convert chest movements into breath counts using specially developed algorithms, according to a Philips press release. The release also states that the device meets all guidelines outlined by the WHO’s Integrated Management of Childhood Illness.
Salim Sadruddin, senior child health advisor at NGO, Save the Children, said the device is a game-changer in effective pneumonia treatment: “If we can remove the subjectivity associated with health workers counting breaths, we can improve the quality of treatment and help improve patient outcomes.”
In addition to quickly diagnosing and treating children in need, experts say the device also has the potential to reduce antibiotic waste and overuse, which can lead to the proliferation of antibiotic-resistant bacteria.
In February, Philips joined the United Nations’ Every Woman Every Child (EWEC) initiative, which aims to improve the lives of three billion people per year by 2025. Since the launch of EWEC in 2010, more than 100 organizations and 40 countries have committed to the project and pledged over $25 billion.
UN Secretary-General Ban Ki-Moon told CNBCAfrica that relief efforts have received a much-needed boost from companies like Philips lending their resources and creative expertise. “The private sector has become eager to increase its engagement, not just because there are business opportunities, but because they see the value in ethical business and helping to improve people’s lives,” he said.
Philips’ device is under consideration for a CE mark, and spokespeople from Philips expect its commercial availability in the second quarter of 2016.
