MIT's Ingestible Device Measures Vital Signs Acoustically
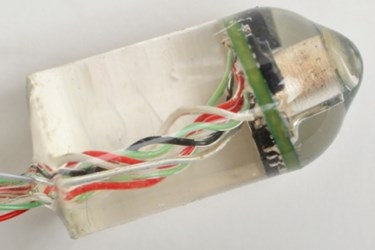
Scientists at MIT have developed an ingestible device that can collect acoustic data and translate it into heart rates and lung sounds. Their proposed system is an alternative to wearable monitoring equipment, which can be cumbersome for patients, particularly trauma victims.
Researchers told MIT News that they’ve essentially designed a tiny microphone encased in a silicone capsule the size of an almond. Once ingested, the sensor can transmit radio signals to an external receiver up to three meters away; the signals then are translated by computer algorithm into readable vital signs. The device, said the researchers, can distinguish between heart and lung sounds and yields consistently accurate results, regardless of how much food is in the digestive tract.
Traditional methods of monitoring vital signs almost always require contact with the skin. Heart rhythms and breathing rates are assessed by doctors using stethoscopes, and continual monitoring requires a system of electrodes attached to the skin with adhesive. These methods can pose a problem if a patient needs to move around or has suffered critical injury to their skin. By measuring vitals internally rather than externally, the MIT team hopes to provide an alternative for patients under these circumstances.
“Trauma patients are a really clear winner here, because you can do vital sign monitoring without touching the skin,” said Albert Swiston, a biomaterials scientist at MIT, to NPR. “You can’t put an electrocardiogram on burn victims if they don’t have skin to apply it to. You need that information, but you can’t touch them.”
In a proof-of-concept study published in PLOS One, authors noted that the technology could “significantly aid telemedicine, optimize performance monitoring of athletes, military service members, and first responders, as well as provide a facile method for rapid clinical evaluation and triage.”
John Rogers, a University of Illinois professor of materials science who was not a part of the research, told MIT News that the capabilities of the proposed system could “provide a powerful complement” to existing monitoring technology.
In the past few years, device developers have introduced a host of ingestible robotic “pills” that perform a variety of different functions — such as PillCam, which takes pictures of the gastrointestinal tract, and the recently FDA-approved sensor developed by Proteus Digital Health that measures medication adherence.
While most ingestible devices currently in development are capable of one specific function, Swiston pointed out that the MIT device was already capable of two, and he imagines future incarnations of the technology that are even more sophisticated. For instance, the device is currently being redesigned to include core temperature monitoring and could one day be used in place of holter monitors to detect heart arrhythmias.
Giovanni Traverso, lead author of the study and gastroenterologist at Massachusetts General Hospital, remarked that further research could develop a capsule capable of detecting pathogens in the body, which would trigger a release of antibiotics.
“This development provides the foundation for that kind of system down the line,” said Traverso.
