Medtronic Engineers Remotely Monitor Neurostimulator Implantation
By Jof Enriquez,
Follow me on Twitter @jofenriq
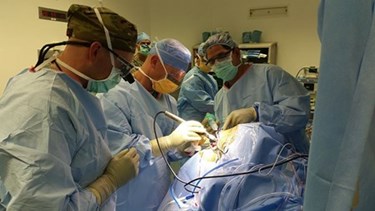
Medtronic engineers recently witnessed, via live video feed, implantation of their company's neuromodulation devices by one of the top neurosurgeons in the United States. The first-of-its kind collaboration is intended to promote better development of devices designed to treat pain and other symptoms caused by nerve disorders, brain tumors, Parkinson's disease, head trauma, and spine injuries.
Steven Falowski, MD, of St. Luke's University Health Network based in Bethlehem, Pennsylvania, implanted Medtronic spinal cord stimulators (SCS), deep brain stimulators, and pain pumps while Medtronic engineers watched the procedures remotely, in real-time, over a live feed from Minneapolis, Minn., according to a report from News Medical.
The report says Dr. Falowski is the Lehigh Valley region’s only fellowship-trained, functional neurosurgeon, as well as a national leader in neurostimulation implantation, having implanted 150 neurostimulators annually and nearly 50 deep brain stimulators last year.
“We’ve been working to develop this capability for several years,” Dr. Falowski said in a press release from St. Luke's. “The live feed, which includes a specialty camera attached to my surgical headlamp, allows Medtronic engineers to see a surgery from my visual perspective and allows the engineers to see exactly how I am using their equipment.”
During surgery, the engineers were able to ask questions as Dr. Falowski implanted the medical devices. The feedback will help engineers make modifications in design and function in future devices.
Patient privacy is maintained during the live feed as the camera covers only the operative field and the patient is completely draped.
“The technology at St. Luke’s is really on the leading edge, especially for neuromodulation. As a result of this partnership, St. Luke’s patients will have access to new technology before any other health care center in the country,” Dr. Falowski says in the release.
According to St. Lukes, Dr. Falowski is one of a handful of physicians in the country with pre-market release access to new medical devices up for approval by the Food and Drug Administration (FDA). This, along with the surgeon's standing relationship with Medtronic, has contributed to the success of their neurostimulation program at the health network.
A report from Lehigh Valley Business cites Dr. Falowski's experience as a major reason that St. Luke’s was one of 20 U.S. hospitals chosen to take part in an ongoing clinical study by St. Jude Medical Inc., where the company's Prodigy with Burst therapy SCS devices were implanted in patients to relieve both chronic pain and paresthesias.
