FDA Updates Guidance On Ethnicity And Race Data In Clinical Trials
By Jof Enriquez,
Follow me on Twitter @jofenriq

The U.S. Food and Drug Administration (FDA) has issued updated guidance for sponsors collecting and reporting race and ethnicity data in submissions for clinical trials for medical devices, drugs, and biologics.
The updated guidance supersedes a 2015 guidance, and has been made more consistent with current standards set by federal mandates and agencies, particularly the Office of Management and Budget (OMB), Directive 15; the 2010 Affordable Care Act (ACA); the Department of Health and Human Services (HHS) Implementation Guidance on Data Collection Standards for Race, Ethnicity, Sex, Primary Language, and Disability Status; and the FDA Safety and Innovation Act (FDASIA), Section 907 Action Plan.
The Action Plan – based on an FDA report that showed inconsistencies with race and ethnic data collection by sponsors – sought to improve the quality and completeness of demographic subgroup data, to identify barriers to clinical trial enrollment of subgroups, and to make data more transparent, said RAPS. FDA says the updated guidance supports the Action Plan by providing recommendations on how best to collect race and ethnicity data.
Specific to medical devices, FDA recommends application sponsors collect ethnicity and race data in accordance with the OMB recommendations, the information collection standards discussed in the guidance document and, when finalized, the Center for Devices and Radiologic Health (CDRH) and the Center for Biologics Evaluation and Research (CBER) Age/Race/Ethnicity draft guidance document.
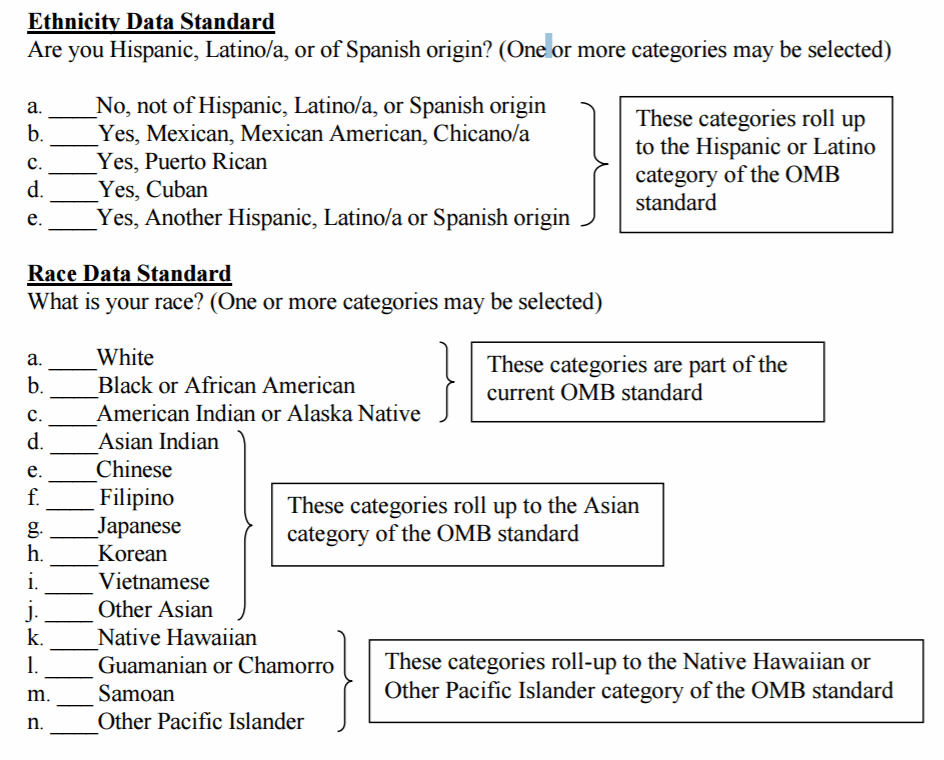
In general, for sponsors of clinical studies conducted both inside and outside the United States, FDA recommends:
1. Using a two-question format. Example:
- Question 1 (answer first): Do you consider yourself Hispanic/Latino or not Hispanic/Latino?
- Question 2 (answer second): Which of the following five racial designations best describes you? More than one choice is acceptable.
2. Let trial participants self-report race and ethnicity information, including designating a multiracial identity.
3. For ethnicity: participants should have the following minimum choices:
- Hispanic or Latino: A person of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin, regardless of race. The term, “Spanish origin,” can be used in addition to “Hispanic or Latino.”
- Not Hispanic or Latino
4. For race: participants should have the following choices (including offering an option of selecting one or more racial designations or additional subgroup designations).
- American Indian or Alaska Native
- Asian
- Black or African American
- Native Hawaiian or Other Pacific Islander
- White
FDA discourages using the term "non-white" and instead recommends using more detailed race and ethnic categories by geographic region.