FDA Issues Update on Quality System Inspections Reengineering
The mission of the reengineering effort was to develop an inspection program that results in more focused and efficient inspections. The effort should help FDA investigators focus in on key manufacturing and quality areas at the manufacturer during inspections, in order to determine their state of compliance with the Quality Systems Regulation.
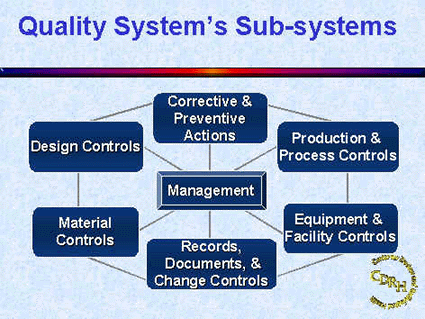
While the QSIT approach to inspections was derived from the theory that there are seven sub-systems in the quality system regulation (21 CFR, Part 820), four primary areas were chosen to focus the inspection. The four top subsystems chosen for the QSIT inspection are Management Controls, Design Controls, Corrective and Preventive Actions (CAPA) and Production and Process Controls. The remaining three subsystems are covered via "linkages" within the QSIT Guide.
In order to incorporate QSIT into the inspection process, a compliance program was developed. A draft document entitled "Draft Compliance Program Guidance Manual: Inspection of Medical Device Manufacturers" that explains the use of the QSIT was published in summer of 1999. This compliance program also provides guidance on three other inspection programs: Medical Device Reporting (MDR), Corrections and Removals, and Tracking. The QSIT Guide provides guidance on inspecting those programs in addition to the QS/GMP inspection guidance. Sterilization inspection guidance was also developed and placed into the QSIT Guide.
Send comments to the Quality Systems Inspections Reengineering Team, 2094 Gaither Rd., HFZ-300 Rockville, MD 20850; Tel: (301) 594-4616 (Tim Wells, Team Leader); E-mail: trw@cdrh.fda.gov.
Edited by Ursula Jones
