FDA Issues UDI Guidance Tailored For Small Device Makers
By Nick Otto
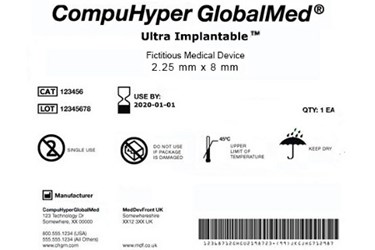
The FDA recently issued guidance advising small medtech companies on how to comply with the agency’s unique device identification (UDI) system, which requires device makers to label their products with codes aimed at improving product recalls, organizing electronic health records, and deterring counterfeiting efforts.
The guidance provides small businesses with instructions on how to label devices so they conform to the final rule, which was issued last September and went into effect shortly after. It contains specific information on key definitions, compliance dates, and formatting requirements related to the UDI system to help smaller device makers understand and remain compliant with the final rule.
The two main parts to the UDI system include a label requirement and a data submission requirement. Every medical device label and every device package must include a UDI, unless it has been granted an exception or alternative.
A UDI is generally composed of a device identifier (DI) and a production identifier (PI).
The DI is a mandatory, fixed portion of the UDI that identifies, among other things, the manufacturers as the labeler and the specific model or version of the device.
A PI is a conditional, variable portion of the UDI, which includes information such as the serial number of a specific device or the device’s expiration.
Every UDI must be presented in two forms:
- Easily readable plain-text
- Automatic identification and data capture (AIDC) technology. If the AIDC technology is not evident upon visual examination of the label or package, the label or package must disclose the presence of the AIDC technology.
If the device is Class I, the guidance says a universal product code (UPC) will be acceptable as the UDI on the device label and package.
Earlier this year, the FDA provided additional information for device makers seeking exemption from the rule, providing direction on how to request an exemption and information on permissible alternative identification processes.
The recent guidance is considered a level 2 guidance, and although it is intended to be implemented immediately, industry is still allowed to submit comments.
