FDA Approves Headband Device To Prevent Migraines
By Joel Lindsey
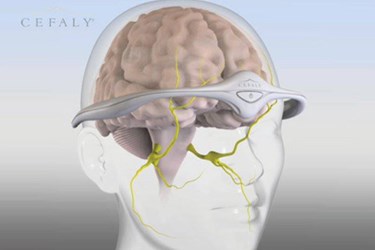
The FDA recently granted market approval for a new wearable medical device designed to prevent migraines.
The device, called Cefaly, is a plastic band that patients can position over the tops of their ears, wrapping it across their foreheads just above the eyes. A self-adhesive electrode attaches to the center of the forehead, and the device sends an electrical current into the skin and the underlying tissues in order to stimulate certain areas of the trigeminal nerve, which many believe is associated with the onset of migraine headaches.
Cefaly Technology, a company located in Belgium, manufactures the device.
“Cefaly provides an alternative to medication for migraine prevention,” Christy Foreman, director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health, said in a statement released by the agency. “This may help patients who cannot tolerate current migraine medications for preventing migraines or treating attacks.”
The FDA granted its approval after reviewing a clinical study conducted in Belgium. During the study, 67 patients — who had experienced more than two migraines per month and who had not taken any preventive medication for three months prior to using Cefaly — reported experiencing “significantly fewer days with migraines per month” while using the device.
Additionally, the agency cited a patient satisfaction study of 2,313 Cefaly users in France and Belgium, 53 percent of whom reported satisfaction and a desire to purchase the device for continued use.
Currently, the device has been approved in the U.S. for prescription use in patients 18 years or older. According to the FDA, no serious side effects have been reported, although it is recommended that the device should only be used for 20 minutes a day. Clinical studies also indicate that the device did not completely prevent migraines, nor did it reduce the intensity of the migraines that did occur.
While Cefaly Technology has yet to reveal purchasing details for the U.S. market, the device currently sells for around $300 in Canada.
“This device is a promising step forward in treating migraine headaches, as it addresses an important part of what we believe triggers and maintains a migraine attack.” Dr. Myrna Cardiel, a clinical associate professor of neurology at NYU Langone Medical Center, said in an article published by CNET News. She also indicated that a 53 percent patient satisfaction rating is similar to “most oral migraine preventive medications.”
According to the FDA’s press release, Cefaly gained FDA approval “through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk medical devices that are not substantially equivalent to an already legally marketed device.”
In an in-depth series of articles published earlier this year by Med Device Online, Dr. Michael Drues described the de novo classification option as an “alternative to the lengthy premarket approval process” that “has never quite caught on among device makers.”
Image Credit: Cefaly
