3D-Printing Technology Drives Medtech Innovation
By Myles Whiting, Owen Mumford Ltd.
The global healthcare regulatory landscape is complex and evolving, driven primarily by a desire for ever-improving patient outcomes and safety.1 The medical device industry also is seeing change driven by longer life expectancies, increased regulatory scrutiny, and healthcare reform.
And, over the past 60 years, rapid evolution in available technologies, particularly in the medical industry, have led to continuous advancements in the physical and technological nature of medical devices.
These advancements have allowed medical device manufacturers to evolve from simpler solutions. This evolution has led tweaks, like the replacement of some glass components with plastic, to more advanced revolutions, such as robotics and automatic assembly lines. In the last two years, we’ve seen the introduction of 3D printing technologies.
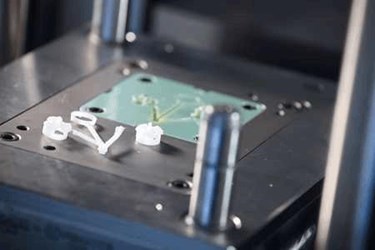
The introduction of 3D printing to the industry has empowered doctors, researchers, and medical device manufacturers to work fast, test thoroughly, and customise like never before. Manufacturers now can test product concepts faster during the prototyping stages of product design, and at the same time increase the level of confidence in a design.
Until two years ago, we outsourced this prototyping, which would take significant amounts of time. Still, despite advances in 3D printing technology and the scale of its components, we were restricted by difficulty in identifying and obtaining the appropriate materials.
To overcome this obstacle, we adopted a hybrid tooling approach, where we completely transformed our prototyping process. The 3D printer works alongside an injection moulding machine, which results in the ability to produce prototypes overnight, in the desired material. This approach meant we could avoid the long turn-around time and costly process to deliver the product when outsourcing.
This process has been taken a step further by standardising the insert sizes, meaning the whole design team can use the 3D printer as a prototyping tool, only having to worry about getting the cavity dimensions right.
According to Gartner’s Hype Cycle for 3D printing 2015,2 medical products are leading some of the most significant new developments in 3D printing technology. Gartner predicts that within the next two to five years, 3D-printed internal and external medical devices will be in mainstream use.
The report states that 3D-printed prototyping will continue to quickly mature, seeing widespread adoption. Fast-forward ten years, and 3D printing could be at a stage where it’s possible to mass manufacture medical devices with the correct materials.
In some industries, 3D printing already enjoys mainstream use. However, due to the level of control, quality, and testing that is required within a medical environment, it is highly unrealistic that individuals (i.e., the home user) will be 3D printing life-saving medical devices in the home any time soon. Currently, the process requires state-of-the-art manufacturing, clean room production, and inspection and testing facilities to ensure safe, conforming products are produced.
Once 3D-printing technology has evolved to the point where it matches all the requirements in terms of scalability and materials available, there’s the potential to 3D print medical devices at mass scale. However, under current medical device regulations, there always will be a need for a high level of control and testing to ensure patient safety.
Additionally, the Deloitte Healthcare and Life Sciences Predictions 20201 report points to connected healthcare, the rise of bio-sensing devices, and in-home care — versus clinics and hospitals — as industry drivers. The report suggests a lessening of the barriers between diagnostics, drugs, and devices for each specific therapy area, affecting the way combination products are regulated and developed.
In order to stay ahead, manufacturers need to be constantly innovating, using newer and faster technologies to provide solutions that make a difference to the end user. The critical nature of this need places, consequently, increased importance on human factors engineering. Through the use of human factors engineering, manufacturers can improve self-management through intuitive medical device design, making adherence easier for the end-user.
About The Author
 Myles Whiting, Product Design Team Manager at Owen Mumford, holds a BSc in Product Design and has worked in the medical device industry for 13 years. As a product design and human factors expert, he also is a member of BSI, developing relevant standards relating to human factors and drug delivery.
Myles Whiting, Product Design Team Manager at Owen Mumford, holds a BSc in Product Design and has worked in the medical device industry for 13 years. As a product design and human factors expert, he also is a member of BSI, developing relevant standards relating to human factors and drug delivery.
References
- Taylor, K., Ronte, H. and Hammett, S. (2014). Healthcare and Life Sciences Predications 2020 A bold future?. Deloitte LPP. [online] Available at: http://www2.deloitte.com/content/dam/Deloitte/global/Documents/Life-Sciences-Health-Care/gx-lshc-healthcare-and-life-sciences-predictions-2020.pdf
- Shanler, Michael, and Pete Basiliere. Hype Cycle For 3D Printing, 2015. Gartner [online] available at: https://www.gartner.com/doc/3100228/hype-cycle-d-printing-
