Barcoding Standards And The Search For A UDI "Easy Button"
By Greg Bylo, GS1 US
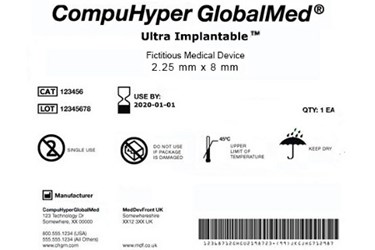
The healthcare industry is preparing for Sept. 24 — the U.S. Food and Drug Administration’s (FDA) next deadline for the unique device identification (UDI) of Class II devices, a category that includes x-ray machines and surgical needles.
With each UDI deadline comes a rush of déjà vu for the medical device community. While many companies have prepared for these compliance dates and are well on their way toward UDI success, many have yet to start and are looking for a quick fix. However, UDI implementation requires long-term vision, and the process provides an opportunity for companies to adopt more efficient business practices that save money, as well as ensure compliance.
Embrace Good Manufacturing Practices
Typically, the companies that are further along in their UDI implementation also are up-to-date with Good Manufacturing Practices (GMPs), Six Sigma, Lean manufacturing, or other operational structures designed to create efficient manufacturing environments. Barcoding or identifying products via GS1 standards often are essential components of these practices.
GS1 standards are the most widely-used supply chain standards in the world. The U.P.C. bar code — just one example of a GS1 standard — is scanned more than five billion times a day globally. These information standards provide the accurate identification and communication of information regarding products, assets, services, and locations globally in order to drive supply chain visibility and efficiency. They help companies authenticate products and allow them to trace products throughout each stop in the supply chain. GS1 — along with Health Industry Business Communications Council (HIBCC) and ICCBBA — has been selected as an official issuing agency supporting the FDA UDI rule.
The reason many companies may be delaying UDI implementation is because they view the new regulations as something an organization can address quickly, and then return to business as normal. That’s not the case, particularly if the basics of GMPs and GS1 standards implementation have not been achieved, or even initiated. This procrastination can create problems in working environments where employees are asked to serve in various cross-functional roles due to budgetary constraints.
To succeed with UDI implementation, organizations need to view UDI as a process, not just a single item to check off of the to-do list. UDI implementation involves an all-encompassing plan, one that should be headed by a designated project owner who will work with regulatory, engineering, and operations — all aspects of the company.
Prioritize Master Data Management
It is a common misconception that UDI is simply about product labeling. At its core, UDI implementation also is about master data management. It’s critical for manufacturers to understand supply chain standards’ vital role in UDI labeling, as well as their importance in mastering the upload of required information to the FDA’s Global Unique Device Identification Database (GUDID).
To date, more than 650,000 devices have been uploaded to the GUDID, and the next two UDI compliance dates are expected to push millions more products into the database. For the system to achieve its goal of accurately tracing products from source to provider or patient, standards must adapt to sustain the consistent exchange of electronic data.
GS1 standards are designed to enable that master data management and high level of traceability. Manufacturers typically start GS1 adoption by identifying their products marked with a barcode containing a Global Trade Item Number (GTIN), which uniquely identifies that product. Once products have been identified with GTINs and corresponding bar codes for global uniqueness, electronic data exchange can be achieved. Product GTINs then can be loaded to the GUDID, satisfying that requirement of the FDA’s UDI rule. Additionally, this digital exchange of standardized product information further automates business processes, reducing errors and saving time by eliminating manual data entry. Business transactions become seamless, empowered by the ability to link internal systems to an external system that all trading partners can utilize.
Furthermore, implementation of GS1 or comparable standards reduces redundancies in supply chain processes, improving overall risk management and crisis preparedness, all by providing a foundation for master data management. Such standards also allow healthcare companies to more effectively leverage information technology and to become more data-driven.
Take Decisive, Immediate Action
For those device manufacturers that are behind in their UDI implementation, it’s important to move forward — fast. And, while no true “easy button” exists for UDI implementation, there typically are three viable approaches, dependent upon budget and resources.
Outsourced Implementation – Some companies hire an outside consultant and operate under a “general contractor” approach to UDI implementation. This consultant likely is experienced in UDI regulations and can complete the entire task.
Limited Partner Implementation – This approach entails seeking help from experts in specific areas of UDI implementation and relying on them for strategic advice and guidance. For example, you might decide to turn to an issuing agency to better understand the process of obtaining UDI tracking codes, but buy the necessary equipment and handle the printing yourself.
Do It Yourself – The DIY approach can be a challenge if you are not an FDA rule expert. Coming up to speed on all facets of the UDI rule can be a daunting task, but FDA provides resources that can help. You’ll have to understand and implement supply chain standards, as well as upload your UDI tracking codes to the GUDID. Other considerations include purchasing and operating the technologies that will allow you to print the UDI on your medical device.
As a final word of advice, it is important to keep in mind the spirit of the rule and make every effort to achieve the goal of enhanced patient safety. By 2020, the healthcare industry should be well on its way to tracking medical devices with the same ease we track items ordered through our favorite e-commerce platforms. It’s up to each manufacturer to play their part in this industry-wide journey that will sustain safer supply chain practices well into the future.
About The Author
Greg Bylo is VP of Healthcare at GS1 US, leading GS1 Healthcare US, an industry-wide collaboration between pharmaceutical, medical device, provider, and patient care professionals to drive the adoption of GS1 Standards for improved patient safety and supply chain performance in the healthcare industry. Greg works closely with industry stakeholders to understand their collective objectives and challenges—particularly as they relate to UDI and the Drug Supply Chain Security Act. He can be contacted at gbylo@gs1us.org.
